A) They represent the same compound.
B) They represent different compounds that are constitutional isomers.
C) They represent different compounds that are geometric isomers.
D) They represent different compounds that are alkenes.
E) They represent different compounds that are alkanes.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which sequence ranks the following isomers in order of increasing boiling points? 
A) 2<1<3
B) 2<3<1
C) 3<1<2
D) 3<2<1
F) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
What two hybrid atomic orbitals overlap to form the C-C s bond in acetonitrile, CH3C≡N?
Correct Answer

verified
Correct Answer
verified
Short Answer
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 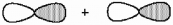
Correct Answer

verified
Correct Answer
verified
Short Answer
What two hybrid atomic orbitals overlap to form the C-C s bond in allene, H2C=C=CH2?
Correct Answer

verified
Correct Answer
verified
Essay
Provide the condensed formulas of three structural isomers with molecular formula C5H12 and arrange them in order of increasing boiling point.
Correct Answer

verified
C(CH3)4 < (C...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the approximate CCC bond angle in the compound below? 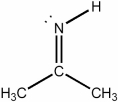
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
The HCH bond angle in propane (CH3CH2CH3) is ________.
Correct Answer

verified
approximat...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Are the two compounds shown below best described as or 
Correct Answer

verified
constituti...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What intermolecular forces are present among molecules in dimethyl ether, CH3OCH3?
A) London forces only
B) hydrogen bonding only
C) both London dispersion forces and hydrogen bonding
D) both London dispersion forces and dipole-dipole forces
F) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Vildagliptin is a recently released antidiabetic drug (J. Med. Chem. , 7902).
Circle and name each functional group in vildagliptin. 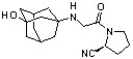
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a constitutional isomer of a compound with an empirical formula C3H7O and a formula mass of 118.164?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Anisole, the compound shown below, is an example of ________. 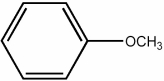
A) an ester
B) an ether
C) an alcohol
D) an aldehyde
E) a ketone
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
The CCC bond angle in allene (H2CCCH2) is ________.
Correct Answer

verified
Correct Answer
verified
Essay
Draw the structure of any hydrocarbon alkene which contains 4 carbon atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many carbon-carbon s bonds are present in the molecule shown? 
A) 1
B) 2
C) 3
D) 4
E) 5
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following does not contain a carbonyl group?
A) aldehyde
B) ketone
C) carboxylic acid
D) ester
E) ether
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The HNC bond angle in the cation [CH2NH2]+ is approximately ________.
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the hybridization of oxygen in acetaldehyde (CH3CHO) and estimate the OCH bond angle.
Correct Answer

verified
The hybridization of...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the hybridization of the nitrogen atom in the molecule below? 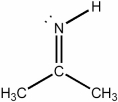
A) s
B) sp
C) sp2
D) sp3
E) none of the above
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 129
Related Exams