Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the term below which best describes the geometry of acetylene (HCCH) .
A) trigonal bipyramidal
B) trigonal
C) tetrahedral
D) square planar
E) linear
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
The molecule shown below contains ________ pi bonds and ________ sigma bonds. 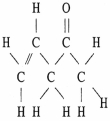
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following functional groups have at least one sp2 hybridized carbon atom as a constituent of the group?
A) carboxylic acid
B) alkene
C) aldehyde
D) ether
E) ester
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the condensed structures of two structurally isomeric amines that contain two carbons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Triethylamine [(CH3CH2) 3N] is a molecule in which the nitrogen atom is ________ hybridized and the CNC bond angle is ________.
A) sp2, >109.5°
B) sp2, <109.5°
C) sp3, >109.5°
D) sp3, <109.5°
E) sp, 109.5°
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Explain why the free rotation about the carbon-carbon bond in CH3CH3 is not present in CH2CH2.
Correct Answer

verified
The single carbon-carbon sigma bond pres...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which atomic orbital combination would result in a molecular sigma bond?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 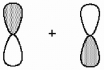
Correct Answer

verified
Correct Answer
verified
Essay
What intermolecular attractions exist in a pure sample of methylthiol, CH3SH?
Correct Answer

verified
London dispersion fo...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Would you expect sodium chloride (NaCl) to be highly soluble in the organic solvent hexane (CH3CH2CH2CH2CH2CH3)? Briefly explain your answer.
Correct Answer

verified
One would  expect NaCl to be highly solu...
expect NaCl to be highly solu...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Choose the correct hybridization for the atom indicated in the molecule below.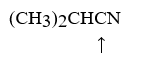
A) sp
B) sp2
C) sp3
D) none of the above
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule below is an ether?
A) CH3CH2OCH2CH3
B) (CH3) 2CHCH2OH
C) (CH3) 2CHCH2NH2
D) (CH3) 2C=CH2
E) CH3CH2CH2CO2H
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the hybridization of oxygen in dimethyl ether (CH3OCH3) and estimate the COC bond angle.
Correct Answer

verified
The hybridization of...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw the structure of any hydrocarbon alkane which contains 5 carbon atoms.
Correct Answer

verified
CH3CH2CH...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Choose the correct hybridization for the atom indicated in the molecule below. 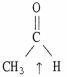
A) sp
B) sp2
C) sp3
D) none of the above
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
What two hybrid atomic orbitals overlap to form the C-C s bond in acetaldehyde, CH3CHO?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many π bonds are present in the molecule shown? 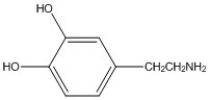
A) 0
B) 1
C) 3
D) 4
E) 6
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Are the two compounds shown below best described as , or 
Correct Answer

verified
constituti...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Does the C – O bond in methanol (CH3OH) possess an individual bond dipole moment? Briefly explain your answer.
Correct Answer

verified
Yes, the C– O bond in methanol...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 61 - 80 of 129
Related Exams