A) a minimum number of electrons has unpaired spins.
B) a minimum number of electrons has intrinsic angular momentum.
C) a maximum number of electrons has unpaired spins.
D) a maximum number of electrons first fills the next energy level.
E) the maximum number of electrons has the same set of quantum numbers.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Zeke says that the magnitude of the orbital angular momentum in the hydrogen atom has the value L = 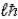 ) Ruth says that the maximum magnitude of the projection of the angular momentum along the direction of a constant magnetic field vector
) Ruth says that the maximum magnitude of the projection of the angular momentum along the direction of a constant magnetic field vector 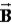 Is
Is 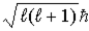 ) Which one,if either,is correct,and why?
) Which one,if either,is correct,and why?
A) Ruth,because the maximum value of L is ![]() .
.
B) Ruth,because the orbital angular momentum always lines up with a magnetic field so that ![]() has its maximum value along the field.
has its maximum value along the field.
C) Zeke,because the maximum magnitude of ![]() is L =
is L = ![]() .
.
D) Zeke,because the orbital angular momentum always lines up with a magnetic field so that ![]() has its maximum value along the field.
has its maximum value along the field.
E) Neither,because they have interchanged the maximum magnitude of ![]() ,
, ![]() ,and
,and ![]() ,its maximum projection along a magnetic field direction.
,its maximum projection along a magnetic field direction.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Suppose a beam of electrons is incident on a collection of hydrogen atoms,all of which are in the lowest energy state (n = 1).What is the minimum energy the electrons can have if they are to excite the hydrogen atoms into the n = 2 state?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Adam and Eve are contemplating the beauty of the hydrogen atom.Adam claims that the quantum states with a given value of the principal quantum number n can have any value of the orbital quantum number 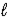 ) Eve says that the Snake told her that a state with a given value of
) Eve says that the Snake told her that a state with a given value of 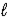 Could have any value of n.Which one,if either,is correct,and why?
Could have any value of n.Which one,if either,is correct,and why?
A) Adam,because the man is always right.
B) Adam because n ≤ ![]() − 1.
− 1.
C) Eve,because n ≤ ![]() − 1.
− 1.
D) Eve,because ![]() ≤ n − 1.
≤ n − 1.
E) Neither,because Adam is wrong and the Snake told a subtle lie.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Light is emitted by hydrogen atoms in the visible range for a hydrogen atom.Its wavelength is 656 nm.What value of n is associated with the light? (RH = 1.097 × 107 m−1)
A) 5
B) 2
C) 4
D) 3
E) 6
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All quantum states forming a shell have the same
A) principal quantum number n.
B) orbital quantum number ![]() .
.
C) orbital magnetic quantum number ![]() .
.
D) n, ![]() and
and ![]()
E) n and ![]() only.
only.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An electron is moving at a speed of 2.1 × 106 m/s in the first Bohr orbit.Determine its de Broglie wavelength.
A) 0.30 × 10−10 m
B) 1.7 × 10−10 m
C) 0.50 × 10−10 m
D) 3.5 × 10−10 m
E) 1.5 × 10−10 m
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
The energy difference between the upper and lower levels in a certain laser is 1.9593 eV.What is the wavelength of the light emitted by the laser?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A hydrogen atom is in its first excited state (n = 2) .The linear momentum of the electron is (in kg ⋅ m/s)
A) 3 × 10−24
B) 2 × 10−24
C) 1 × 10−24
D) 4 × 10−24
E) 3 × 10−15
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following states,5s,3p,4f,5p,4g,3d,and 2p,the one which is NOT allowed is
A) 3p
B) 4f
C) 3d
D) 4g
E) 2p
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The s,p,d,f,symbols represent values of the quantum number
A) ms
B) n
C) ![]()
D) ![]()
E) mj
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The K,L,M symbols represent values of the quantum number
A) n
B) ![]()
C) ![]()
D) ms
E) mj
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All quantum states forming a sub-shell have the same
A) principal quantum number n.
B) orbital quantum number ![]() .
.
C) orbital magnetic quantum number ![]() .
.
D) n, ![]() and
and ![]()
E) n and ![]() only.
only.
G) B) and E)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
How fast is the electron moving in the first Bohr orbit?
A) 3.3 × 106 m/s
B) 2.2 × 106 m/s
C) 4.4 × 106 m/s
D) 5.5 × 106 m/s
E) 5.5 × 1015 m/s
G) C) and D)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
In the subshell of the Li2+ ion with orbital quantum number 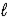 ,the allowed values of the magnetic quantum number
,the allowed values of the magnetic quantum number 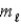 Are
Are
A) − ![]() to
to ![]()
B) −( ![]() + 1) to (
+ 1) to ( ![]() + l)
+ l)
C) −( ![]() + 2) to (
+ 2) to ( ![]() + 2)
+ 2)
D) −( ![]() + 3) to (
+ 3) to ( ![]() + 3)
+ 3)
E) 0 to n − 1
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An electron in a hydrogen atom makes a transition from the n = 3 to the n = 1 energy state.Determine the wavelength of the emitted photon (in nm) .
A) 1006
B) 209
C) 306
D) 103
E) 821
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The allowed values of 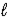 For the n = 3 shell in a Li2+ ion are
For the n = 3 shell in a Li2+ ion are
A) 1,2
B) 0,1
C) 0,1,2
D) 0,1,2,3
E) 1,2,3
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a completely filled atomic shell,
A) the intrinsic spin of the electrons does not produce a resultant magnetic moment.
B) the orbital motion of the electrons does not produce a resultant magnetic moment.
C) the atom will be an alkali metal.
D) only (a) and (b) are correct.
E) none of the above are correct.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose Bohr had chosen the potential energy of the electron in the hydrogen atom to be V = 0 when the electron is in the orbit with n = 1.He could do this by
A) choosing n = 1 for the orbit where the kinetic energy of the electron is zero.
B) adding a constant 13.6 eV to the potential energy for all values of n.
C) adding a constant 27.2 eV to the potential energy for all values of n.
D) subtracting a constant 13.6 eV from the potential energy for all values of n.
E) subtracting a constant 27.2 eV from the potential energy for all values of n.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ground state configuration of chlorine (Z = 17) is
A) 1s2 2s2 2p5 3s2 3p6
B) 1s2 2s2 2p6 3s2 3p5
C) 1s2 2s2 2p6 3s2 3p4 3d1
D) 1s2 2s2 2p6 3s2 3p5 4s1
E) 1s2 2s2 2p6 3s1 3p7
G) None of the above
Correct Answer

verified
B
Correct Answer
verified
Showing 1 - 20 of 59
Related Exams