A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the best product for the following reaction. 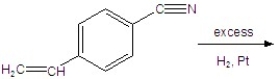
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Provide a series of synthetic steps by which 2-bromo-4-nitrobenzoic acid can be prepared from toluene.
Correct Answer

verified
1) HNO3, H2S...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following is the best method for preparing m-chloroaniline from benzene?
A) NH3; Cl2/AlCl3
B) Cl2/AlCl3; NH3
C) Cl2/AlCl3; HNO3/H2SO4; Sn/HCl, HO-
D) HNO3/H2SO4; Cl2/AlCl3; Sn/HCl, HO-
E) HNO3/H2SO4; Sn/HCl; HO-; Cl2/AlCl3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the electrophile that attacks the aromatic ring during sulfonation?
A) HSO3+
B) SO2+
C) HSO3-
D) H2SO4
E) HSO4-
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the structure of the major organic product of the following reaction. 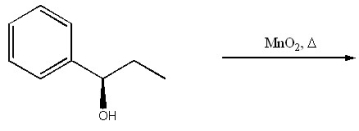
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide the best name for the organic compound below. 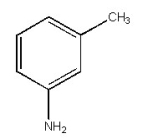
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What specific electrophile is attacked by benzene when it undergoes nitration?
A) HNO3
B) NO3
C) NO
D) NO2
E) NO2+
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the structure of the major organic product of the following reaction. 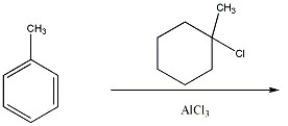
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a correct statement concerning the Friedel-Crafts acylation of benzene?
A) An alkyl group substitutes for a hydrogen.
B) The benzene ring attacks an acylium ion.
C) The acylium ion is resonance stabilized.
D) The acylium ion is often produced from an acyl chloride.
E) More than one equivalent of Lewis acid must be used.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the structure of 3-phenylpentane? 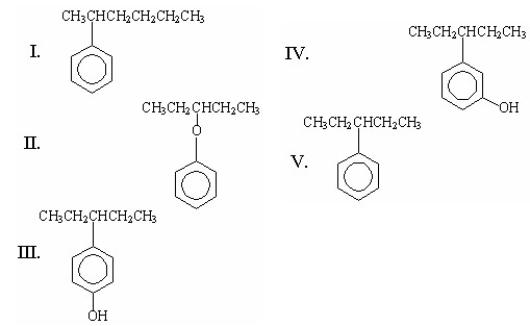
A) I
B) II
C) III
D) IV
E) V
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the structure of styrene? 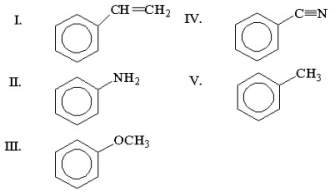
A) I
B) II
C) III
D) IV
E) V
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Explain why nitrobenzene can be used as a solvent for Friedel-Crafts alkylation.
Correct Answer

verified
Because of the deact...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What purpose does FeCl3 serve in the electrophilic aromatic substitution reaction between chlorine and benzene?
A) It serves as a radical initiator to produce the chlorine radical needed to propagate the chain reaction.
B) It functions by destabilizing the carbocationic intermediate and thereby increases the rate of H+ loss.
C) It serves as a Lewis base catalyst by reacting with Cl2 to generate chloride ions.
D) It functions by destabilizing the benzene through formation of a π-complex.
E) It serves as a Lewis acid catalyst by reacting with the Cl2 and thereby activates it toward attack by benzene's π electrons.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the product(s) for the following reaction. 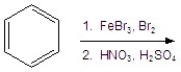
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the major product for the reaction. 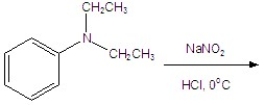
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Give the product(s)for each step of the following reaction. 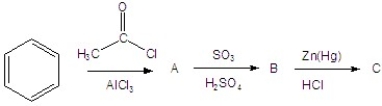
Correct Answer

verified
Correct Answer
verified
Essay
Provide the major organic product of the reaction shown below. 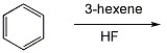
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substrates is an electron donating group overall?
A) -Br
B) ![]()
C) -OCH3
D) ![]()
E) -CCl3
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the major organic product of the following. 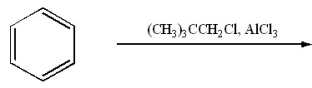
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 155
Related Exams