Correct Answer

verified
Correct Answer
verified
Essay
Provide the equation for the equilibrium constant, Keq, for the following equilibrium. 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which sets of curved arrows accounts for the protonation of propene with HI? 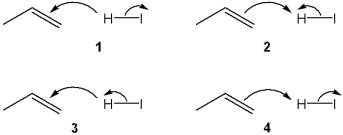
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Use curved arrows to show the movement of pairs of electrons in the following acid-base reaction and show the structures of the conjugate acid and conjugate base. 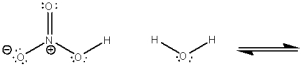
Correct Answer

verified
Correct Answer
verified
Essay
Use curved arrows to show the movement of pairs of electrons in the following reaction between a Lewis acid and a Lewis base, and show the structure of the product. 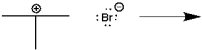
Correct Answer

verified
Correct Answer
verified
True/False
The following correctly shows the electron flow for the given reaction. 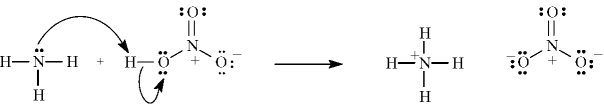
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species is the conjugate acid in the following acid-base reaction? 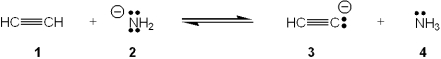
A) 1
B) 2
C) 3
D) 4
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following terms describes the role of ethanol in the acid-base reaction shown? 
A) Brønsted-Lowry acid
B) Brønsted-Lowry base
C) Lewis acid
D) Lewis base
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values? CH3OH > CH3NH2 > CH3CH3 16 38 51
Correct Answer

verified
This trend is best understood in terms o...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
A reaction where G is positive is _____________.
Correct Answer

verified
Correct Answer
verified
Essay
What is the value of the equilibrium constant for the following equilibrium? 
Correct Answer

verified
log10Keq = pKa(acid) - pK...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following statements is not true?
A) "The position of the equilibrium for an exergonic reaction favors products"
B) "The products of an exergonic reaction have a higher Gibbs free energy than the reactants."
C) "The equilibrium constant of a reaction for which G = 0 is 1."
D) " G = H -T S "
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
In the following list, there are two organic Lewis bases. 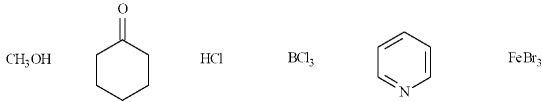
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest pKa?
A) NH3
B) H2O
C) HCl
D) CH4
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 94 of 94
Related Exams