A) H2O
B) HBr
C) NH3
D) CH4
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the strongest acid?
A) CH3COOH
B) FCH2COOH
C) ClCH2COOH
D) BrCH2COOH
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is easiest to deprotonate?
A) CH4
B) CH3CH3
C) CH2=CH2
D) HC CH
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the lowest pKa?
A) H2O
B) H2S
C) H2Se
D) H2Te
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest pKa?
A) SiH4
B) H2S
C) PH3
D) HCl
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) CH3CH3
B) CH3NH2
C) CH3OH
D) CH3F
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
A species that lies at an energy maximum during an individual step in a reaction is termed ____________________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Under which of the following conditions will a reaction be spontaneous when H > 0?
A) "T S = 0"
B) "T S > H "
C) "T S < 0 "
D) " G = 0"
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Complete the equation below for the protonation of 2-butene with HBr. Show the movement of pairs of electrons with curved arrows and provide the structures of the conjugate acid and conjugate base. 
Correct Answer

verified
Correct Answer
verified
Essay
Use curved arrows to show the movement of pairs of electrons in the following acid-base reaction and show the structures of the conjugate acid and conjugate base. 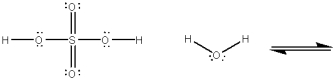
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate pKa value of acetic acid?
A) -7
B) 5
C) 16
D) 51
F) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery. 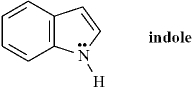 Indole can be classified as a Brønsted-Lowry base but not as a Lewis base..
Indole can be classified as a Brønsted-Lowry base but not as a Lewis base..
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
Which acid has the strongest conjugate base?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate pKa value of HCl?
A) -7
B) 5
C) 16
D) 51
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the value of the equilibrium constant, Keq, for the following reaction? 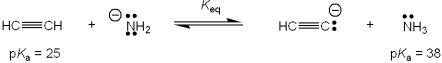
A) "1013"
B) "10-13"
C) "13"
D) "1/13"
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) CH3OH
B) CH3CHO
C) CH3COCH3
D) CH3COOH
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the equation that relates the equilibrium constant, Keq, to the acid dissociation constant, Ka, for the following equilibrium. 
Correct Answer

verified
Correct Answer
verified
True/False
In the following list, there are four Lewis acids, CH3OH, HCl, BCl3 and FeBr3. 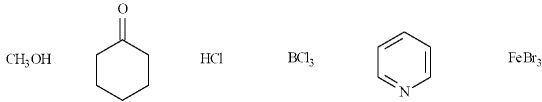
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
Which acid will be almost completely deprotonated by NaOH?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the strongest acid?
A) CH4
B) CH3CH3
C) H2C=CH2
D) HC CH
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 94
Related Exams