A) 4
B) 5
C) 6
D) 7
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has the highest electronegativity?
A) C
B) P
C) Si
D) Cl
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true regarding resonance structures?
A) Each resonance structure is in rapid equilibrium with all of the other structures
B) The resonance structures may have different energies
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must have the same number of electrons
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true regarding resonance structures?
A) All resonance structures must have the same number of electrons
B) Each atom in all of the resonance structures must have a complete shell of valence electrons
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must be valid Lewis structures
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species possesses a formal charge?
A) CCl4
B) SiCl4
C) AlCl4
D) PCl3
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the aldehydes that have the formula C5H10O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate value of the length of the C=C bond in ethane, CH2=CH2?
A) 121 pm
B) 134 pm
C) 142 pm
D) 154 pm
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in ethyne (acetylene, HC CH). Label each bond (e.g., C-H bond) and indicate which atomic orbitals contribute to this bond (e.g., C 2sp3 + H 1s).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate C-C-C bond angle in propyne, HC CCH3?
A) 90
B) 109
C) 120
D) 180
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds has the smallest dipole moment?
A) C-N
B) C-O
C) C-F
D) O-H
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds is the most polar?
A) O-H
B) C-H
C) C-C
D) H-H
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in ethene (ethylene, H2C=CH2). Label each bond (e.g., C-H bond) and indicate which atomic orbitals contribute to this bond (e.g., C 2sp3 + H 1s).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a primary (1 ) alcohol? 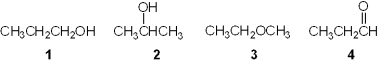
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the C=O bond of acetone, (CH3) 2C=O?
A) C 2sp3 + O 2sp2
B) C 2sp2 + O 2p
C) C 2sp2 + O 2sp2
D) C 2sp3 + O 2sp
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true about the carbonate anion, CO32-?
A) All of the oxygen atoms bear the same amount of charge
B) All of the carbon-oxygen bonds are the same length
C) The carbon atom bears the negative charge
D) It is basic
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The hybridization on the numbered carbon atoms in the following compound would be Carbon 1 sp3 and Carbon 2 sp2. 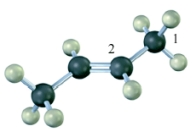
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The curved arrows in the resonance structure for the acetate ion shown below 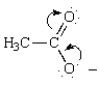 indicate the following alternative resonance structure for the acetate ion.
indicate the following alternative resonance structure for the acetate ion. 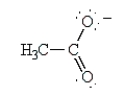
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an carboxylic ester? 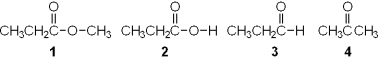
A) 1
B) 2
C) 3
D) 4
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a ketone?
A) CH3CH2COOH
B) CH3CH2CHO
C) CH3CH2CH2OH
D) CH3COCH3
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-carbon molecular bonding orbital of ethyne, HC CH?
A) C2p + C2p
B) C2sp + C2sp
C) C2sp2 + C2sp2
D) C2sp3 + C2sp3
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 115
Related Exams