A) Brønsted-Lowry acid
B) Brønsted-Lowry base
C) Lewis acid
D) Lewis base
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Provide the equation for the equilibrium constant, Keq, for the following equilibrium.

Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which sets of curved arrows accounts for the protonation of propene with HI? 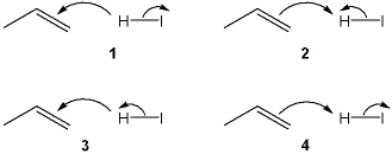
A) 1
B) 2
C) 3
D) 4
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Use curved arrows to show the movement of pairs of electrons in the following acid-base reaction and show the structures of the conjugate acid and conjugate base.
Correct Answer

verified
Correct Answer
verified
Essay
What is the value of the equilibrium constant for the following equilibrium?

Correct Answer

verified
log10Keq = pKa(acid) - pK...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following is the strongest acid?
A) CH3NH2
B) CH3PH2
C) CH3OH
D) CH3SH
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct order of decreasing basicity (stronger base > weaker base) ?
A) NH3 > MeNH2 > H2O > HF
B) MeNH2 > NH3 > MeOH > CH4
C) NH3 > Me3N > H2O > MeOH
D) CH3COONa > NaOH > NaOMe > NaNMe2
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) CH3CH3
B) CH3NH2
C) CH3OH
D) CH3F
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a feature of a Lewis base?
A) proton donor
B) proton acceptor
C) electron pair donor
D) electron pair acceptor
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide the equation that relates the acid dissociation constant, Ka, to the pKa of an acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the value of the equilibrium constant, Keq, for the following reaction? 
A) 109
B) 10-9
C) 9
D) (1/9)
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values?

Correct Answer

verified
This trend is best understood in terms o...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values?
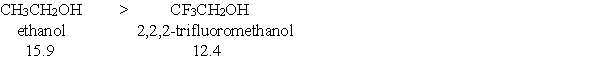
Correct Answer

verified
This trend is best understood in terms o...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following energy diagrams represents the fastest reaction? 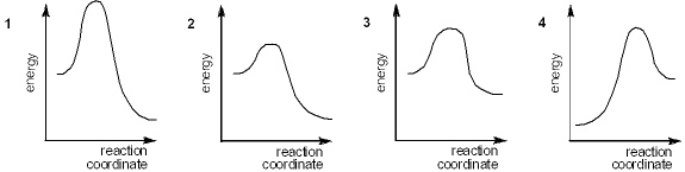
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the strongest acid?
A) CH4
B) CH3CH3
C) H2C=CH2
D) HCºCH
F) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 61 - 75 of 75
Related Exams