A) CH4− ⋅ + 2 e−
B) CH3− + H⋅
C) CH4+⋅ + 2 e−
D) CH3⋅ + H+ + 2 e−
F) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Short Answer
What is the most likely molecular formula of a compound that has a molecular ion in the EI mass spectrum with m/z of 97 and an M+1 peak that is 6.6% of the intensity of the molecular ion peak?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In mass spectrometry, which of the following phrases is shortened to the acronym "FAB"?
A) flight averaged bumping
B) fast atom bombardment
C) fragmented atom beam
D) flying atom bashing
F) B) and C)
Correct Answer

verified
B
Correct Answer
verified
Short Answer
Dehydration of the following compound will produce a peak at a m/z of _______. 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gives rise to a prominent peak in the mass spectrum at m/z of 39?
A) 1-octyne
B) 4-octyne
C) 5-methyl-3-octyne
D) 2,2-dimethyl-3-heptyne
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the terms with their descriptions. -Sample is bombarded with photons obtained from a laser source
A) EI-MS
B) FAB
C) MALDI
D) ESI-MS
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not prominent in the mass spectrum of 2-methyl-2-butanol?
A) M − 29
B) M − 18
C) M − 15
D) M − 12
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the most common products of the fragmentation of a molecular ion?
A) two radicals
B) an anion and a cation
C) a radical and a cation
D) two cations
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s). 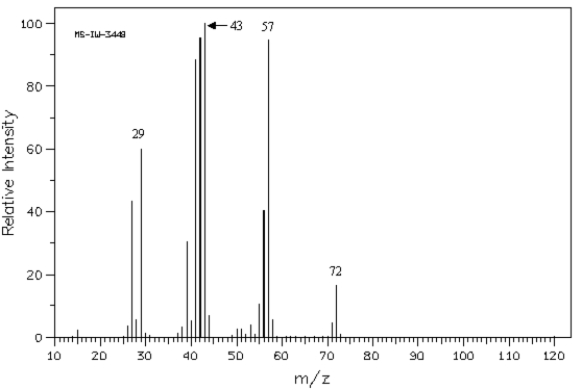 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
-The peak at a m/z of _____ represents the following species.
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
-The peak at a m/z of _____ represents the following species.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the terms with their descriptions. -Sample is bombarded with high-energy electrons
A) EI-MS
B) FAB
C) MALDI
D) ESI-MS
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gives rise to a prominent peak in the mass spectrum with m/z of 45?
A) 1-hexanol
B) 2-hexanol
C) hexanal
D) hexanoic acid
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
This compound contains C, H, and one other atom. The other atom from the mass spectrum is __________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the molecules gives rise to a molecular ion with an odd value of m/z?
A) C6H12Cl2
B) C7H10N2
C) C8H13N
D) C8H14Br2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following does not give rise to a prominent M − 41 peak in the mass spectrum?
A) 1-hexene
B) 3-methyl-1-hexene
C) 4-methyl-1-hexene
D) 5-methyl-1-hexene
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
Mass spectrometry and infrared spectroscopy are complementary techniques because mass spectrometry provides information about the molar mass and formula while infrared spectroscopy helps identify the functional groups in the formula.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass spectral analysis of toluene showed a base peak at m/z = 91. The base peak is because of _____.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A mass spectral analysis of 3-methyl-1-pentamine showed a base peak at m/z = 30. The base peak is because of _____.
A)
B)
C)
D)
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the compound(s) that will undergo McLafferty rearrangement during a mass spectral analysis. 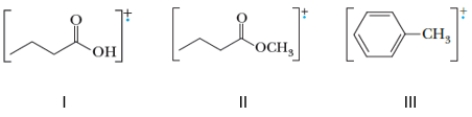
A) Only I
B) Only II
C) Only I and II
D) Only II and III
F) A) and C)
Correct Answer

verified
C
Correct Answer
verified
True/False
Consider the following mass spectrum 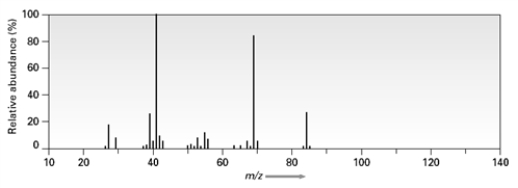 C5H12O might have been used to produce this spectrum.
C5H12O might have been used to produce this spectrum.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the ions "X" and "Y" in the given reaction. 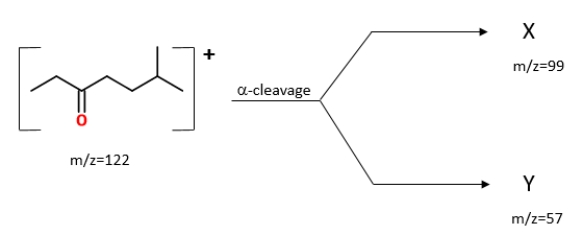
A) X is![]() , and Y is
, and Y is![]() .
.
B) X is![]() , and Y is
, and Y is![]() .
.
C) X is![]() , and Y is
, and Y is![]() .
.
D) X is![]() , and Y is
, and Y is![]() .
.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 75
Related Exams